Infectious bronchitis (IB) is one of the major diseases that frequently affects chickens. Caused by the coronavirus (genus Gammacoronavirus, family Coronaviridae, order Nidovirales), this disease has persisted over the past several years and continues to cause significant economic losses in the poultry industry.
Current Situation of IB
How is the current situation of IB disease spread in Indonesia? Based on data collected by the Surveillance Analyst team of Medion throughout 2024, IB disease cases identified through clinical symptoms and anatomical pathology changes during necropsy (chicken dissection) were reported in a total of 302 cases. This figure represents a 19.8% increase compared to the 252 IB cases reported in the previous year.
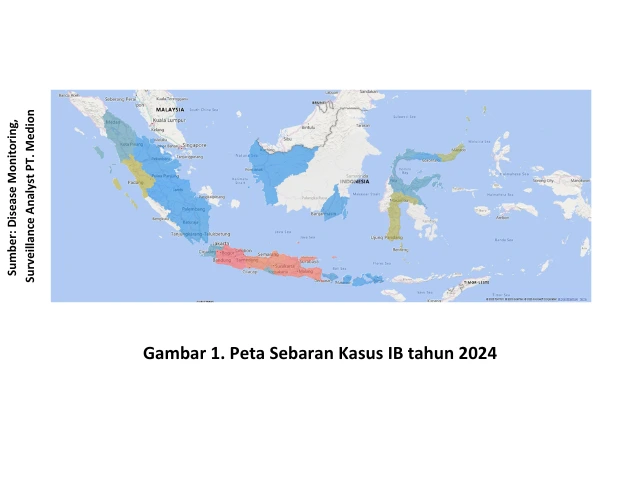
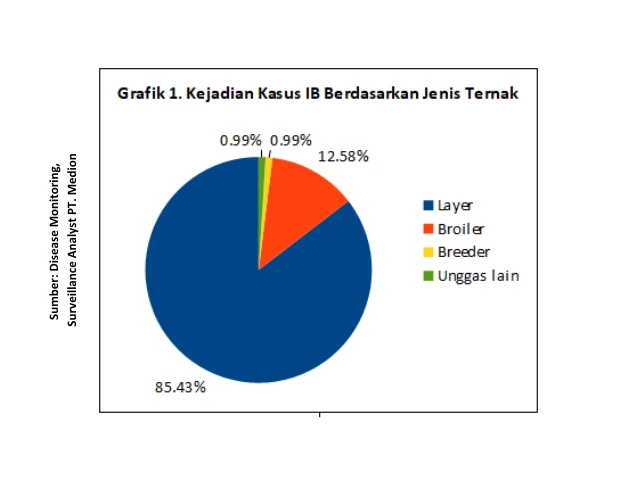
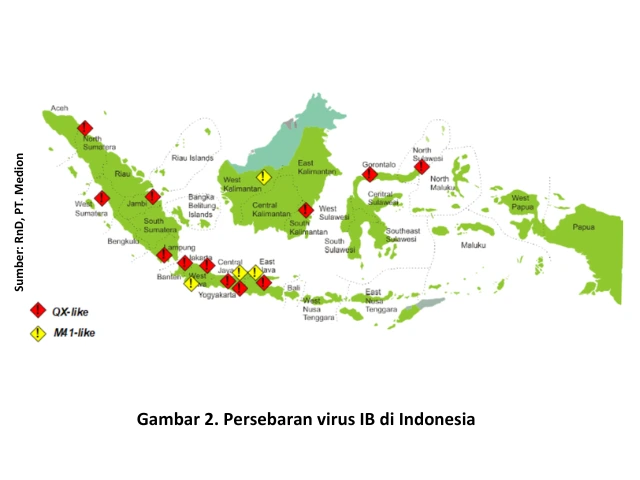
According to data from the Surveillance Analyst team of Medion, IB disease cases in Indonesia have spread almost evenly across the country. The highest incidence rates were recorded in West Java, Central Java, and East Java, marked in red on the distribution map shown in Figure 1, indicating a high number of cases. The occurrence of IB disease is predominantly found in layer chickens, accounting for 85.43%, followed by broiler chickens at 12.58% (Figure 1).
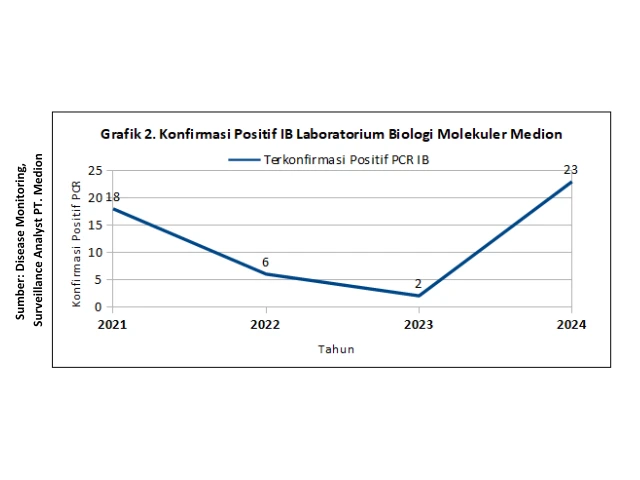
The number of confirmed positive IB cases from samples tested at the Medion laboratory using PCR (polymerase chain reaction) continues to be detected each year, with a significant increase in confirmed positive IB cases observed in 2024 compared to the previous three years (Figure 2).
The coronavirus primarily acts as a pathogen of the respiratory system. This virus can replicate in the epithelial cells of the upper respiratory tract of chickens, causing respiratory symptoms such as gasping, coughing, sneezing, and nasal discharge [3].
However, beyond that, the IB virus can also infect other organ systems such as the urinary, reproductive, or digestive systems, depending on the strain or genotype of the infecting IB virus [5]. Different IB virus strains have varying abilities to infect specific organ systems according to their target tissues (tissuetropism).
For example, the classic M41 strain (Massachusetts) primarily attacks the respiratory system, the variant QX-like strain affects the digestive, urinary, and reproductive systems, while the 4/91 (793B) strain targets the respiratory, urinary, and muscular systems [12].
The IB virus has the ability to mutate or exchange genetic material easily. As a result, many variant strains have been identified, making them difficult to control through vaccination. In addition to the classic strain such as M41-like (Massachusetts), several variant IB virus strains have also been detected in Indonesia, including the QX-like strain originating from China and the 4/91 (793B) strain from the United Kingdom [13].
In line with this information, mapping of the IB virus conducted by the Research & Molecular Biology Development team of Medion (2015–2024) showed that the dominant IB viruses infecting poultry in Indonesia are the M41-like strain and the IB variant that, based on molecular biology characterization, belongs to the same group as the QX strain, namely group A2, also referred to as QX-like (Figure 2).
Based on observations by the Technical Education and Consultation team of Medion, current IB virus infections in the field not only affect the respiratory system but also cause damage to the reproductive system, leading to a decrease in egg production ranging from 10–50%, along with reduced egg quality. IB cases in the field generally result in low mortality rates; however, these rates can increase to 20–30% when secondary bacterial infections such as Escherichia coli or Mycoplasma sp. occur [4].Select 13 more words to run Humanizer.
Disease Diagnosis
IB disease causes clinical symptoms that can be categorized into three main forms: respiratory, renal (damage to the urinary system), and reproductive. These manifestations depend on the organ or organ system infected by the IB virus and the tissue tropism of each viral strain or genotype [10].Select 53 more words to run Humanizer.
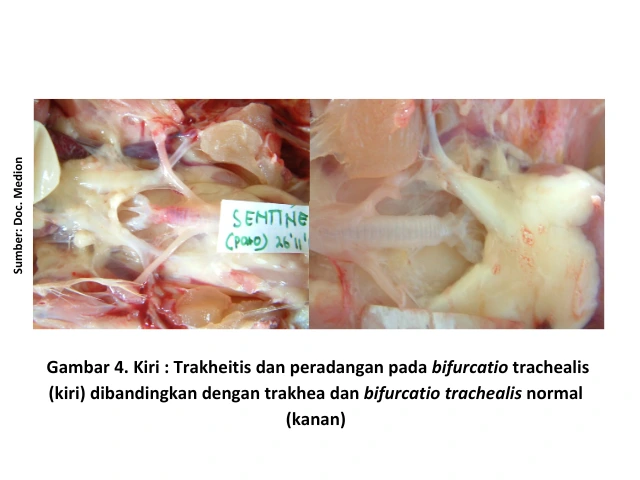
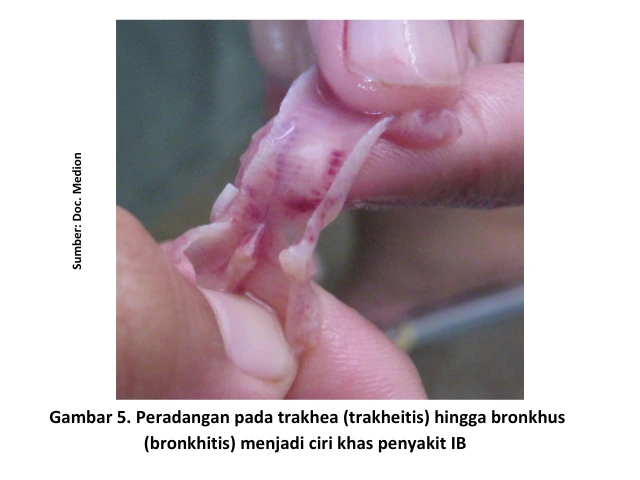
Respiratory symptoms are indicated by nasal discharge, lacrimation (watery eyes), sneezing, coughing, tracheal rales, and gasping [6]. Anatomical changes observed during necropsy of chickens infected with IB showing respiratory symptoms include inflammation of the upper respiratory tract, particularly in the trachea and bronchi.
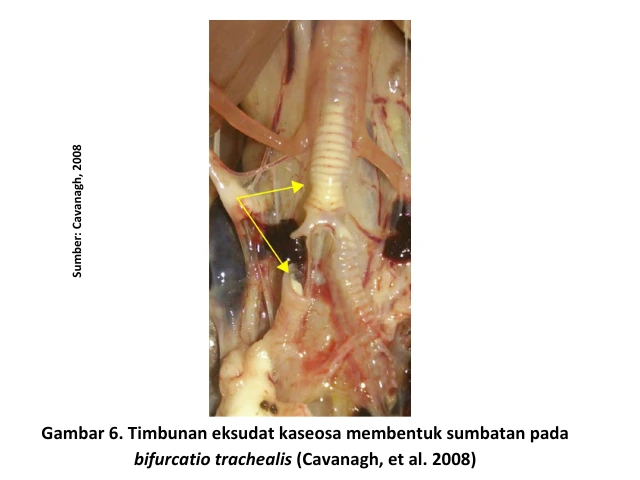
In addition, serous, catarrhal, or even caseous exudates may be found in the trachea. These exudates can cause blockages at the bifurcatio trachealis (the point where the trachea branches into the bronchi), leading to breathing difficulties and oxygen deficiency (asphyxia), which may result in the death of the chicken. When accompanied by secondary bacterial infections, conditions such as pericarditis, perihepatitis, and airsacculitis may also be observed [4].

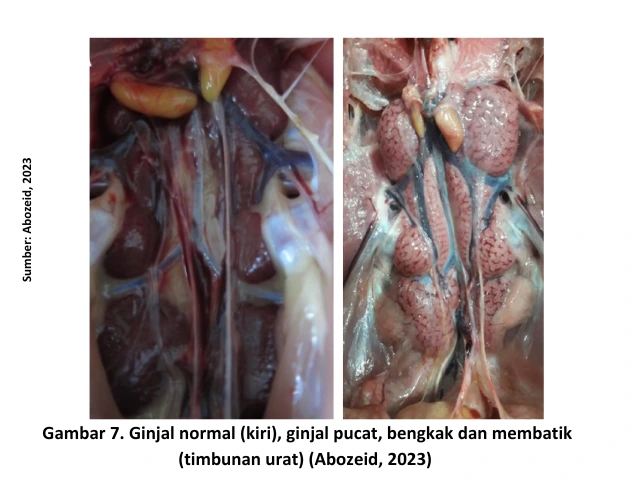
Chickens infected with the renal form of IB are affected by nephropathogenic strains of the IB virus (kidney-damaging), such as 4/91 (793B), QX-like, B1648, Aus T, and TW. Infected chickens exhibit symptoms such as depression, dull feathers, excessive drinking, and wet, whitish feces containing large amounts of uric acid. During necropsy, the kidneys may appear pale and mottled, with swelling and visible uric acid deposits in the ureters [1].
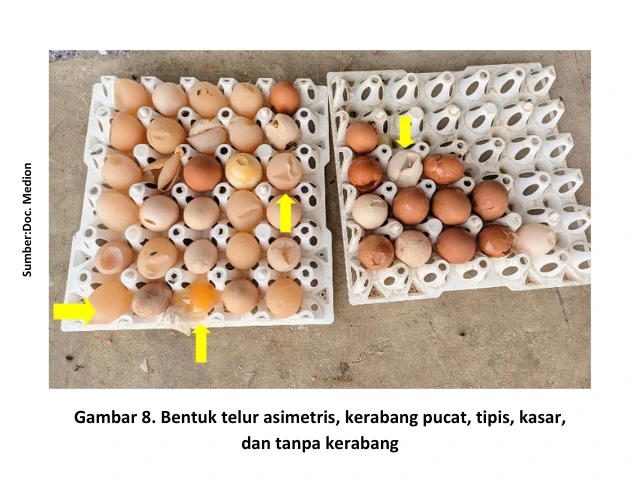
The form of IB disease that affects the reproductive system in layer chickens is characterized by a decrease in egg production accompanied by a decline in egg quality, such as pale eggshells, thin shells, rough shells, shell-less eggs, small-sized eggs, and asymmetrical egg shapes.
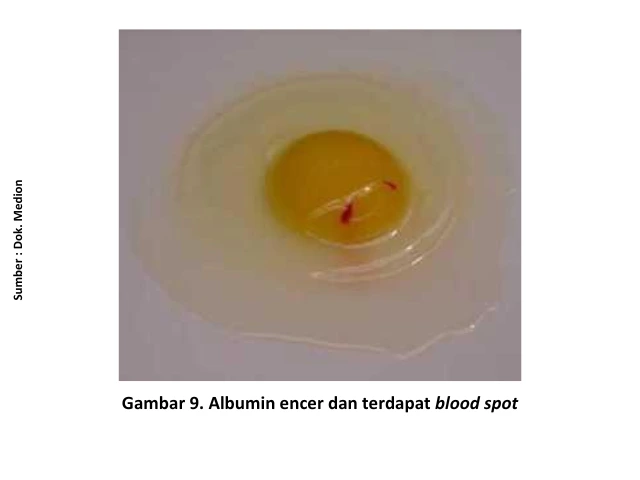
When the eggs are cracked open, a decline in internal egg quality can be observed, such as a thinner albumen (egg white) and the absence of a clear boundary between the thick and thin albumen layers [15]. The reduction in egg production varies depending on the severity of the infection, the viral strain involved, and the immunity status of the chickens [7].
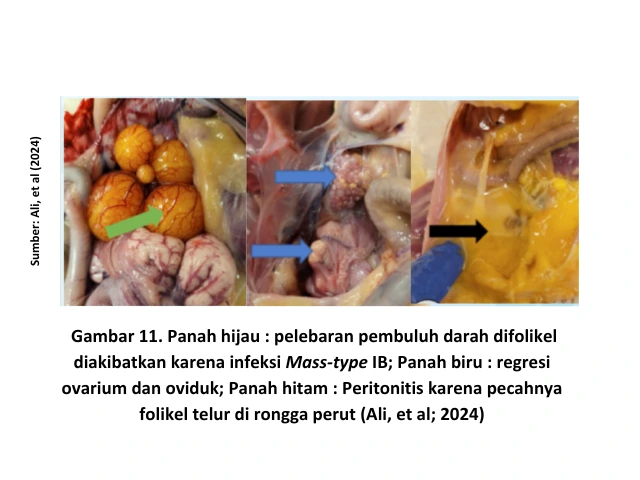
IB variant strains such as QX-like are more pathogenic than Mass-type strains in their ability to damage the oviduct [16]. When the IB virus infects young chickens, the damage it causes to the oviduct becomes permanent. As a result, when these chickens reach the laying phase, they are unable to produce eggs. This condition is commonly known as false layers syndrome [6].
In IB cases caused by the QX strain, anatomical pathology changes include dilation of the oviduct filled with clear fluid (cystic oviduct). Clinically, this condition can be recognized in chronic cases, where the chicken’s abdomen appears enlarged and the bird walks upright with its head raised, resembling a penguin.
In the early stages, clear fluid may accumulate in the oviduct, although it is not yet visible clinically. Egg peritonitis is often observed as a result of shortening and narrowing of the oviduct, causing the yolk to be released into the abdominal cavity [15]. The presence of yolk in the abdominal cavity provides a medium for bacterial growth, leading to inflammation of the peritoneum (peritonitis) [12].

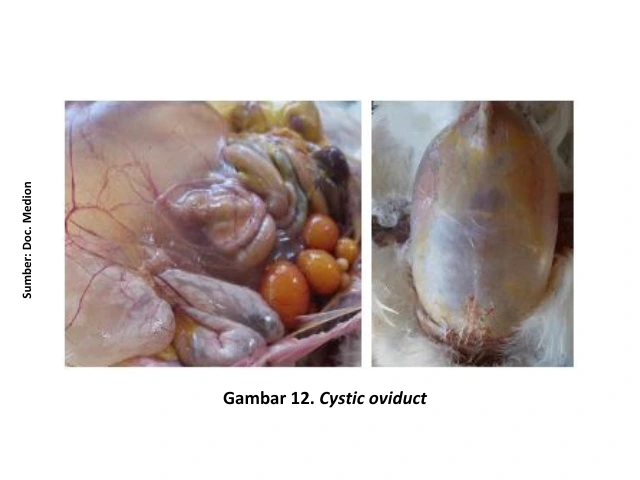
Confirmation of diagnosis can be carried out through laboratory testing based on virus detection or evidence of seroconversion. However, routine vaccination against IB—using either live or inactivated vaccines—can complicate diagnosis through serological testing, as it is not always possible to distinguish between antibodies resulting from vaccination and those from field infections [14].
IB Control Strategies
Since its first discovery in the 1930s, IB disease continues to be detected to this day. The diversity of IB serotypes is primarily due to variations in the spike (S) protein. The S protein on the surface structure of the coronavirus is divided into two subunits: S1 and S2.
The S1 subunit shows amino acid variation among IB virus strains ranging from 20% to 50%. This occurs because the IB virus, like most RNA viruses, is prone to mutation and genetic recombination. Mutations that occur during the viral replication process often go undetected, as the IB virus lacks a proofreading mechanism in its viral RNA polymerase [11].
Currently, there are three types of IB viruses still circulating globally: classic IB (e.g., Massachusetts (M41); Connecticut), variant IB (e.g., 4/91 (793B); A2 (QX-like); CA1737/04), and nephrotropic IB (e.g., Gray; Holte; Australian T). Among these, the variant IB strain QX-like is the main cause of IB cases in layer, broiler, and breeder chickens in Indonesia.
The most effective control of IB disease is through proper vaccination and biosecurity measures. Vaccination using the classic IB Massachusetts strain is widely applied around the world. However, the Massachusetts strain vaccine alone cannot provide adequate protection against the 4/91 (793B) and QX-like strains due to low cross-protection levels [13].
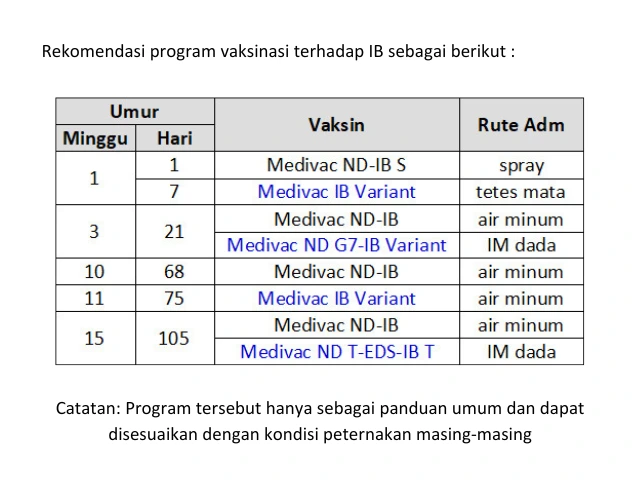
Medion is committed to supporting farmers in preventing IB by providing various vaccines containing both classic and variant IB strains, available in active and inactivated forms. Simultaneous vaccination using both classic and variant IB vaccines offers broad cross-protection against multiple field challenges, including protection from severe clinical symptoms [8].
Medivac IB Variant is an active IB variant vaccine containing the M02 (793B) strain, while Medivac ND G7-IB Variant Emulsion contains a combination of the classic IB virus strain Massachusetts 41 and IB variant viruses that are homologous to current field isolates, namely the M02 (793B) and M01 (QX-like) strains. These vaccines can be used in combination during the production period to provide better protection against IB virus challenges in the field.
There is no specific treatment once chickens are infected with IB, although supportive therapy using antibiotics to control secondary bacterial infections can be very helpful. Secondary infections resulting from IB generally cause more severe problems than IB infection alone [9].
Supportive treatment using the herbal product Reduvir can be applied to help reduce viral replication in tissues. Reduvir is a liquid suspension formulation containing extracts of Rhizoma coptidis and Andrographis paniculata.
The extract of Rhizoma coptidis contains the active metabolite berberine, while Andrographis paniculata contains the active compound andrographolide. Both possess herbal antiviral properties and are capable of enhancing the immune system. Administration of Reduvir is effective in improving poultry health and reducing mortality in birds infected with the IB virus.
References
- Abozeid, H. H. “Global Emergence of Infectious Bronchitis Virus Variants: Evolution, Immunity, and Vaccination Challenges”. Transboundary and Emerging Diseases. Vol. 2023, Issue 1. https://doi.org/10.1155/2023/1144924
- Ali, et al. 2024. “Comparative pathogenicity of CA1737/04 and Mass infectious bronchitis virus genotypes in laying chickens”. Front. Vet. Sci. 11:1338563. doi :10.3389/fvets.2024.1338563
- Cavanagh D. “Coronavirus avian infectious bronchitis virus”, Vet Res. (2007) 38:281-97.doi :10.1051/vetres:2006055
- Cavanagh D., et al. “Infectious bronchitis”. Disease of Poultry. 2008. Iowa, Blackwell Publishing, 117-135.
- Cook JK, et al. “The long view: 40 years of infectious bronvhitis reseach”. Avian Pathol. (2012) 41:239-500. doi:10.1080/03079457.2012.680432
- Crinion R. A. P., “Egg quality and production following infectious bronchitis virus exposure at one day old”, Poultry Science. (1972) 51, no. 2, 582–585, https://doi.org/10.3382/ps.0510582, 2-s2.0-0015301719.
- Dolz R., Vergara-Alert J., Pérez M., Pujols J., and Majó N., “New insights on infectious bronchitis virus pathogenesis: characterization of Italy 02 serotype in chicks and adult hesn”, Veterinary Microbiology. (2012) 156, no. 3-4, 256–264, https://doi.org/10.1016/j.vetmic.2011.11.001, 2-s2.0-84858746649.
- Eid, A.A.M; et al. ”The efficacy of simultaneous successive classic and variant infectious bronchitis virus vaccines versus circulating variant II Egyptian field virus”. Open Veterinary Journal (2024), Vol. 14(1):90-107. doi: 10.5455/OVJ.2024.vI4iI.9.
- Farm Health Online 2018 : https://www.farmhealthonline.com/disease-management/poultry-diseases/infectious-bronchitis/
- Jackwood M. W. and de Wit S., E. D. Swayne, “Infectious bronchitis”, Diseases of Poultry, 2020, Wiley-Blackwell, 167–188.
- Jenkins G. M., Rambaut A., Pybus O. G., and Holmes E. C., “Rates of molecular evolution in RNA viruses: a quantitative phylogenetic analysis”, Journal of Molecular Evolution. (2002) 54, no. 2, 156–165, https://doi.org/10.1007/s00239-001-0064-3, 2-s2.0-0036147047.
- M. Najimudeen, S., H. Hassan, M. S., C. Cork, S., & Abdul-Careem, M. F. (2020). “Infectious Bronchitis Coronavirus Infection in Chickens: Multiple System Disease with Immune Suppression”. Pathogens, 9(10), 779. https://doi.org/10.3390/pathogens9100779
- Wibowo, MH., et al. “Molecular characterization of pathogenic 4/91-like and QX-like infectius bronchitis virus infectiong commercial poultry farms in Indonesia”. Vet World. 2019 Feb 20;12(2):277-287. doi: 10.14202/vetworld.2019.277-287.
- WOAH Terertiral Manual. 2008. Chapter 3.3.2 – Avian infectious bronchitis.
- Zhang X., Liao K., Chen S., Yan K., Du X., Zhang C., Guo M., and Wu Y., “Evaluation of the reproductive system development and egg-laying performance of hens infected with TW I-type infectious bronchitis virus”, Veterinary Research. (2020) 51, no. 1, https://doi.org/10.1186/s13567-020-00819-4, 95.
- Zhang X., Yan K., Zhang C., Guo M., Chen S., Liao K., Bo Z., Cao Y., and Wu Y., “Pathogenicity comparison between QX-type and Mass-type infectious bronchitis virus to different segments of the oviducts in laying phase”, Virology Journal. (2022) 19, no. 1, https://doi.org/10.1186/s12985-022-01788-0, 62.