Bapak Jaya – by email
Are antiprotozoal drugs for coccidiosis therapy resistant in poultry?
Answer:
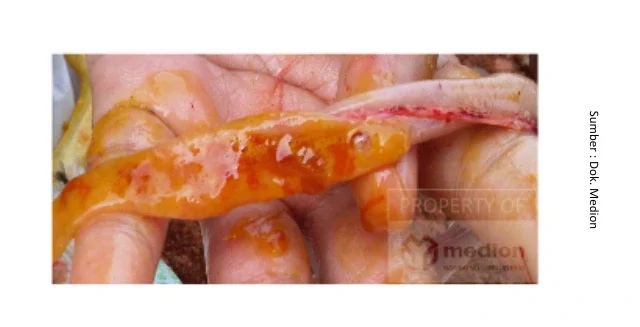
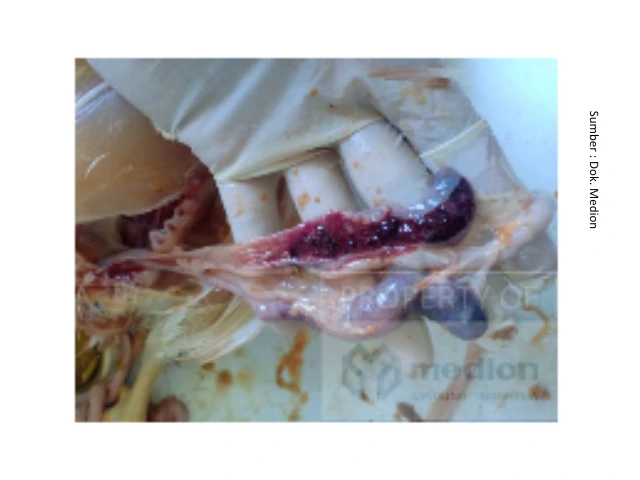
Thank you for the submitted question. Coccidiosis is an infectious disease in poultry, caused by a luminal protozoan agent known as Eimeria sp. Eimeria species that infect birds can cause enteric disease, characterized by bloody diarrhea, weakness, weight loss, dehydration, pallor, dull plumage, and sometimes death. Eimeria sp. those that belong to intracellular parasites will cause disruption of the intestinal mucosa when reproducing, causing tissue damage, thereby disrupting the process of eating and absorption of nutrients. Some of the most common species of Eimeria found in poultry include Eimeria acervulina in the duodenum, Eimeria necratix in the jejunum and Eimeria tenella in the cecum. When necropsy is performed, the intestines of infected birds Eimeria sp. will indicate the presence of inflammation (enteritis), the contents of the intestine will look colored orange up to Red Papaya, as well as the finding of blood in the intestinal lumen.
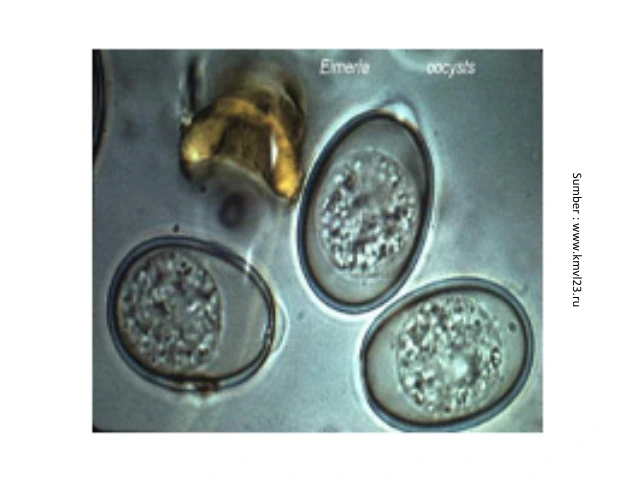

Poultry can be infected Eimeria sp. when swallowing oocysts that are sporulated in the environment and there is a cycle of reproduction of Eimeria in the intestine. Oocysts eaten by chickens will enter the digestive tract, the presence of intestinal peristalsis and digestive enzymes will cause the oocyst membrane to rupture so that the sporocysts inside the oocysts exit into the intestinal lumen. Sporokista mengandung sporozoit yang merupakan tahap infektif dari protozoa (Otranto & Wall, 2025). Sporozoites will enter the intestinal epithelium and begin the reproductive cycle. Sporozoites will penetrate epithelial cells and round off, forming tropozoites that will divide and form schizogony. First generation schizogony will produce first generation merozoites that will damage cells and produce second generation schizogony, which then produces second generation merozoites. This phase is a phase of asexual reproduction Eimeria sp. inside the epithelial cells of the intestine. The sexual phase begins when the merozoites that have managed to penetrate the cell produce male gametophytes (microgametocytes) and female gametophytes (macrogametocytes), which then combine and form new oocysts and are excreted with feces. In this phase, clinical symptoms of bloody diarrhea will appear due to damage to intestinal epithelial tissue (Sokół & Gałęcki, 2018; Swayne, 2020).
Infection Eimeria sp. poultry is a serious problem because it has a significant impact on chicken productivity. In addition to fairly high mortality, other disadvantages such as stunted weight growth, Feed Conversion Ratio Poor (FCR), low uniformity, and the risk of susceptible to other diseases are challenges that must be faced by farmers in dealing with coccidiosis in poultry. Handling coccidiosis in poultry can be done by giving antiprotozoa which is a type of drug to kill Eimeria sp. as the causative agent of coccidiosis and support such as multivitamins to help maintain livestock performance.
However, the current challenge is more than just infection Eimeria sp. in poultry that can disrupt productivity and cause economic losses. Based on disease data collected by the team surveillance Medion, the percentage incidence of coccidiosis disease during the last 3 years in broiler chickens as much as 12.37 % of cases, layer 4.84% of cases, and breeder (layer and broiler) 8.08% of cases. The high incidence of coccidiosis in poultry, followed by the massive use of antiprotozoa raises concerns that antiprotozoal resistance in poultry has an impact on reducing the effectiveness of antiprotozoal drugs against Eimeria sp. Similarly to the issue of antibiotic resistance to bacteria, antiprotozoal resistance will cause protozoa to be resistant to antiprotozoal drugs.
Antiprotozoal resistance is defined as the ability of a protozoan to inhibit the action of an antiprotozoal agent, this phenomenon occurs when the antiprotozoan loses its efficiency and effectiveness to inhibit the growth or kill the protozoan. Elelu et al (2022) in his research explained that antiprotozoal resistance can occur due to natural selection in protozoan agents and selection pressure due to sub-therapeutic doses of antiprotozoa, which causes drug inactivation by resistant genes. Antiprotoza resistance can occur through two main mechanisms: resistance-related mutations in protozoan parasites and selective pressure. Simple, multiple, or quadruple (multiple genetic mutations that occur in four amino acids) in various protozoan genes being a factor that causes antiprotozoal resistance (Capela et al., 2019). The mechanism of resistance through selective pressure is associated with poor diagnosis (including laboratory confirmation) of most protozoal diseases, improper use of antiprotozoal drugs, poor quality of drugs, and non-compliance with standard treatment rules. In addition, the use of sub-therapeutic doses as a prophylactic treatment in animals also contributes to the development of protozoan resistance. Ababu et al (2021) explained that inappropriate antiprotozoal drug use practices have the potential to affect the quality, safety, and effectiveness of antiprotozoal drugs which can lead to the development of drug resistance.
Efforts to prevent antiprotozoan resistance in poultry can be done with the right treatment, by practicing the "5 Principles of proper treatment" including proper diagnosis, proper drug selection, proper dosage, proper application, and timely treatment. In the case of avian coccidiosis, diagnosis is made by looking at clinical symptoms and changes in anatomical pathology. Confirmation of the agent of coccidiosis is carried out by laboratory examination to identify the protozoal agent through microscopic examination of oocysts in feces or intestinal contents. In laboratory tests, the causative agent of coccidiosis will be identified qualitatively by looking at the presence of oocysts and quantitatively by counting the number of oocysts to see the degree of severity of the infection.
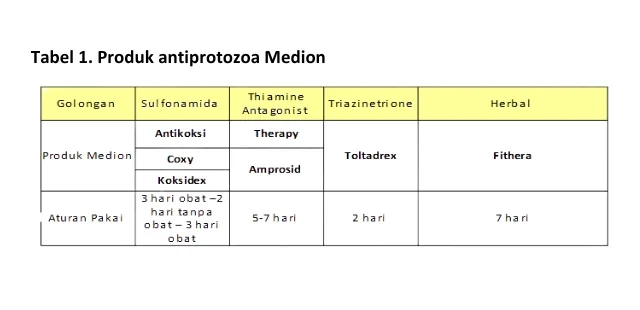
Prevention of antiprotozoal resistance through the appropriate and wise administration of antiprotozoal drugs according to drug manufacturers and animal health personnel will help reduce selective pressure on Eimeria sp. Antiprotozoal drugs used to treat coccidiosis can destroy or inhibit the reproductive ability of protozoa by interfering with metabolic processes, interfering with reproduction, and the neuromuscular physiology of parasites (Capela et al., 2019). Medion, as a veterinary drug manufacturer in Indonesia has antiprotozoal drug products that effectively deal with coccidiosis in poultry. There are three groups of antiprotozoa produced by Medion, namely sulfonamides, thiamine antagonists, and triazinetrione, each group has a different way of working in killing protozoa based on the protozoan life cycle. Golongan sulfonamida dan thiamine antagonist membunuh protozoa pada fase reproduksi aseksual, sedangan golongan triazinetrione membunuh protozoa pada fase seksual dan aseksual. In addition, Medion also produces antiprotozoal herbal medicines that are able to inhibit the multiplication stage Eimeria sp. and prevents sporozoites from penetrating and infecting the walls of the small intestine. The antiprotozoal drugs produced by Medion are presented in Table 1.

Selective pressure on protozoal agents that trigger antiprotozoal resistance can also be minimized by rolling class of antiprotozoa in the handling of cases. Rolling classes will help antiprotozoal drugs remain effective at killing protozoal agents and can suppress the incidence of antiprotozoal resistance. The high incidence of coccidiosis in poultry would be in harmony with the use of antirpotozoa as causative therapeutic agents.
Control of coccidiosis can be done by making preventive efforts through the control of the causative agent by minimizing predesposition factors followed by management control and biosecurity that's good. High cage density and cage humidity, followed by poor litter quality can be a predesposition factor for coccidiosis in poultry because it will create an ideal atmosphere for oocyst sporulation Eimeria sp. Swayne (2020) explains that oocysts Eimeria sp. will sporulate into the infective phase under conditions of oxygen availability and humid and warm environmental conditions for 48-72 hours.
Oocyst Eimeria sp. the one that is sporulated in the environment will trigger the horizontal transmission of coccidiosis. Infected chickens will secrete oocysts along with feces, under suitable environmental conditions for the sporulation process, the oocysts will become infective and can be ingested by healthy chickens. This condition will cause massive transmission of coccidiosis in one population. Therefore, the application of management and biosecurity a good one is essential to prevent infections from occurring. Prevention efforts can be done, among others, by sprinkling lime on the inside and outside of the cage, treating the litter before it is stocked through drying or , formalin to dry, maintain the condition of the litter so that it is not damp by flipping it regularly, replacing the clumping part, and adding new litter when needed. In addition, the optimization of the cage rest period, management brooding period, it is also necessary to regulate the density and ventilation of cages, disinfect cages and equipment, and control the traffic of personnel and vehicles to break the cycle of transmission of coccidiosis.
References
Elelu, N., Agene, G., Sanusi, F., & Al-Mustapha, A. I. (2022). A cross-sectional questionnaire survey on knowledge of anti-protozoal drug use and resistance among AHPs in Kwara State, Nigeria. BMC Veterinary Research, 18(1), 214.
Swayne, D. E. (2020). Diseases of poultry. John Wiley & Sons.
Otranto, D., & Wall, R. (2024). Veterinary parasitology. John Wiley & Sons.
Sokół, R., & Gałęcki, R. (2018). The resistance of Eimeria spp. to toltrazuril in black grouse (Lyrurus tetrix) kept in an aviary. Poultry Science, 97(12), 4193-4199.
Ababu, A., Endashaw, D., Fesseha, H., & Mathewos, M. (2021). Antiprotozoal drug handling and management practices in Asella District, Central Oromia, Ethiopia. Veterinary Medicine International, 2021(1), 6648328.
Capela, R., Moreira, R., & Lopes, F. (2019). An overview of drug resistance in protozoal diseases. International journal of molecular sciences, 20(22), 5748.
Materi Presentasi Khusus_2025_B03_Koksidiosis & NE serta pengendaliannya