The performance of chickens, especially broiler chickens, is greatly influenced by four main factors, namely genetics, environment, nutrition, and management. These factors can affect the growth, productivity, and health of chickens. The implementation of good management, both in terms of maintenance and health, plays a significant role in achieving performance. Maintenance management includes cage rest management, brooding period,, ventilation, litter, water, and feed. Success in the early stages contributes 50% to 90% to the success of chicken farming. Intensive care and attention must be applied during the brooding period because the series of processes that occur in the chicken's body are very important. Failure in the early stages of farming will make it difficult to achieve optimal productivity in the next phase of chicken farming.

The implementation of good health management aims to keep chickens healthy. This can be done through appropriate vaccination programs, supplementation and medication programs, and other programs biosecurity strict. Digestive tract health management is one of the factors for success in health and disease control. Digestive tract health and nutrition are interrelated. As we know, the digestive tract is an organ that plays a role in receiving feed, digesting, absorbing nutrients, and excreting unabsorbed feed residues. Optimal utilization of feed nutrients can only be achieved if the digestive tract is healthy. Several parameters that can be used to assess whether a chicken's digestive tract is functioning properly include:
- Good digestibility and absorption of dietary nutrients
- Very low incidence of illness or mortality due to digestive disturbances
- Feed Convertion Ratio (FCR) (in line with standards)
- Minimal fecal odor
Digestive health is important because it plays a role in nutrient absorption and maintaining tissue function gut-associated lymphoid tissue or GALT. The digestive tract along the small intestine and large intestine contains lymphoid tissue scattered within the epithelium, lamina propria, or in the form of plates peyer’s patches. GALT is part of the lymphoid tissue that functions as a site for mucosal immune responses to produce antibodies and receive mucosal immune response stimuli (Marsetyawan, 1993).
In livestock affected by digestive diseases, the digestive tract, especially the intestinal mucosa, will experience damage that causes nutrient absorption disorders. This results in impacts such as growth disorders, low uniformity, increased FCR, and increased mortality. A healthy digestive tract in chickens can be characterized by a long intestine and large diameter, allowing villi and crypts to develop optimally, thereby maximizing absorption area. Good feed digestion and nutrient absorption are indicated by low FCR values and low mortality rates. Factors influencing digestive organ quality:
- Good initial maintenance management
Immediate feeding upon arrival of chickens, implementation of management brooding period, good, optimal digestive organ growth, optimal absorption of egg yolk.
- Gut microbiota
Maintain a balanced intestinal microflora and prevent pathogenic bacteria from growing excessively. This can be done by administering probiotics, prebiotics, acidifiers, and enzymes.
- Feed quality
Ensure that nutritional needs are met every day in terms of both quality and quantity. Feed ad libitum (especially during the starter), control feeding regularly to ensure chickens receive feed according to their needs. Adjust feed nutrition according to growth phase. Rations containing fungal toxins (mycotoxins) can damage digestive organs such as the intestines, gizzard/gizzard and liver. It is equally important to pay attention to the storage shed and feeding area.
- Minimizing the risk of exposure to disease agents
Implementation biosecurity Good hygiene will reduce the risk of chickens becoming infected with gastrointestinal diseases.
Factors Causing Digestive Health Disorders
Digestive disorders in chickens are caused by various factors, including feed, environmental conditions, and disease. Some of these factors include:
- Quality of feed and drinking water
- Presence of fungi and mycotoxins in feed
- Challenges from digestive diseases (necrotic enteritis, coccidiosis, colibacillosis)
- Balance of intestinal microflora
- Chickens experiencing stress
- Disorders of the digestive immune system
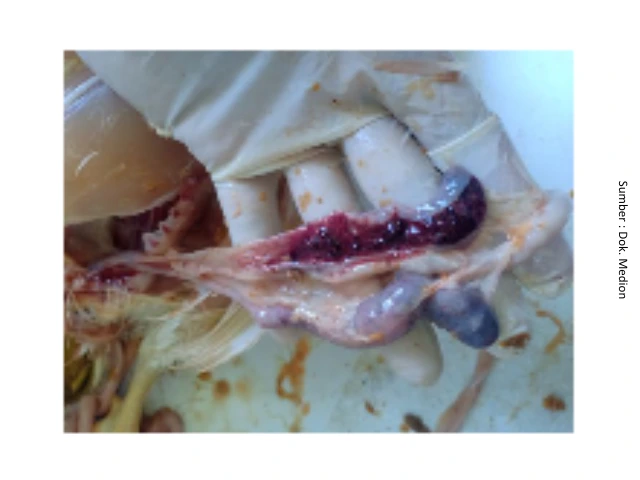
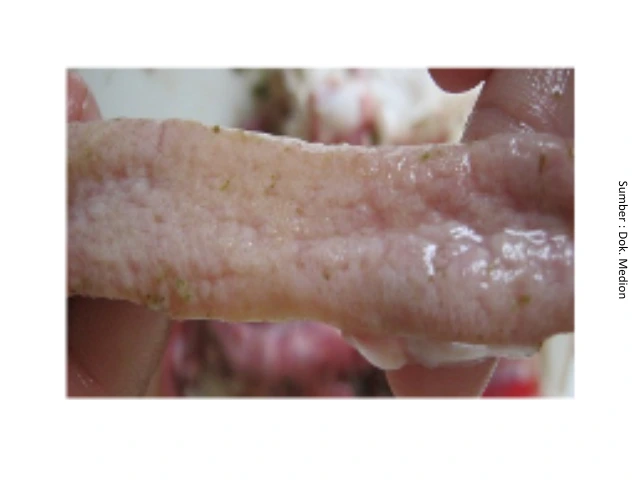
The balance of microflora or bacteria that are naturally present in the intestines is essentially dynamic, depending on the condition of the intestines. In a balanced state, the microflora provides benefits to the host. However, if this balance is disrupted, it can affect the morphology of the digestive tract, lead to the emergence of bacterial infections in the digestive system, and even damage the immune system of chickens. Bacteria that are normally present in the digestive tract of chickens can also cause infections, such as bacteria Clostridium perfringens (causes of NE disease) when chickens are in poor condition and supported by uncomfortable environmental conditions, then outbreak NE can occur. This is triggered by the declining condition of the chicken's body, while the concentration of bacteria continues to increase. High concentrations of bacteria in the intestines can be excreted through feces and can also infect other chickens. These bacteria can cause inflammation and destruction of the intestinal lining. In addition, the bacteria will also produce toxins that can interfere with the absorption of nutrients by the intestines and cause increased intestinal peristalsis, which ultimately leads to symptoms of diarrhea.
Maintaining Chicken Digestive Health
To maintain the digestive health of chickens, there are several things that need to be considered, namely
1. Provide and give rations with the right nutritional value, the right amount of rations, and do not give rations that have clumped together or contain mold. Also pay attention to the condition of the ration storage area in terms of temperature and humidity, and make sure it is safe from rats or other insects. Do this monitor on feed consumption. In addition, periodically rotate the feed to increase appetite.
2. Reducing the population of disease vectors around chickens
- Clean the coop. Coop cleanliness has a huge impact on chicken health and performance. When the coop is clean, the concentration of disease pathogens will decrease, thereby reducing disease challenges and keeping chickens free from digestive diseases.
- After cleaning, continue with liming. Liming the coop is necessary to reduce disease pathogens, one of which is coccidia, the cause of coccidiosis.
- A minimum rest period of 2 weeks is calculated after the cage has been cleaned and disinfected. This is to break the disease cycle.
- Disinfect empty cages with Sporades or Formades. Three days before chicks in, spray the cage and its equipment, including the feed and water containers, again using Medisep.
- Drinking water quality control includes physical (clear, colorless, and odorless), chemical (neutral pH and non-hard), and biological (free from contamination) aspects. E. Coli, Salmonella sp. or other disease-causing microorganisms). Sanitize drinking water (Desinsep) if the water source is positively contaminated E. Bra and other bacteria. Don't forget to always keep drinking areas clean from contamination such as feces and litter.
- Pay attention to temperature, humidity, ventilation, cage density, and quality. litter or husks, especially during the rainy season like now. In management litter, flip it over litter to prevent litter wet. Immediately replace wet and clumped litter. If there is only a small amount of clumping, it can be separated and removed from the cage.
3. Optimizing digestive tract health and maintaining a balanced intestinal microflora. To maintain the health and performance of the digestive system, supplemental products can be provided, for example, feed additive herbal. Herbs are known to contain various nutrients and bioactive compounds that serve as supplements, antibacterials, antiparasitics, and antiprotozoals. Optigrin is feed additive containing herbal ingredients that help maintain the digestive health of poultry, thereby supporting optimal performance. Optigrin It also has antibacterial and antiprotozoal properties, making it effective in suppressing the growth of pathogenic microbes (an alternative to AGPs). Optigrin It can also enhance the function of the body’s defense system against disease agents.
After hatching is the most critical period in a broiler chicken’s life. When chicks emerge from the egg, their digestive and immune systems are still immature, and they are not yet ready to face environmental challenges. There is a transition to air respiration, body temperature regulation, and a shift from yolk lipid nutrition to complex dietary nutrients. These changes are closely related to the physical and functional development of the gastrointestinal tract (GIT) and the maturation of active immunity. As a result, the capacity to digest feed, absorb, and transport nutrients is limited during the early life of broiler chickens. To achieve the genetic potential of modern broilers, neonates must quickly adapt to efficiently digest and utilize nutrients from complex exogenous feed sources, with energy derived primarily from carbohydrates.
The immune system of poultry develops only partially at hatching. The development of this system, particularly gut-associated immunity, responds to early feeding and dietary nutrition and is crucial for protection against exogenous organisms during the first week.
The role of the microbiome—or its absence—in the overall development of newly hatched chicks cannot be overlooked. The complex microbial community in the digestive tract plays a vital role in production by supporting intestinal structural development, digestion, protection against pathogens, and maturation of the host’s immune system. The chick’s digestive tract is sterile at hatch but is quickly colonized by microbiota through feed and the environment. A stable microbiome, with high species diversity and an even distribution of dominant species, appears to form by the third week of life. The intestinal microbiome contains various bacterial species that are heavy consumers of amino acids (AAs) and energy for their growth and colonization. Therefore, the absence or low population of bacteria during the first week may provide an advantage to the host in terms of nutrient utilization.
Growth and Development of the Digestive Tract
After emerging from the yolk sac, chicks must quickly adapt from obtaining their nutritional needs from yolk lipids to utilizing a diet that is primarily carbohydrate-based. To meet the growth and maintenance requirements of rapidly growing chicks, the digestive system must be able to digest and absorb exogenous nutrients at levels sufficient to meet those needs. Consequently, chicks place a high priority on intestinal growth to ensure that the nutrient supply function is fulfilled. In addition to the physical architecture of the GIT, a strong barrier function and a well-developed immune system must be in optimal condition.
The growth of the digestive tract occurs allometrically, with components of the GIT growing at different rates compared to other body parts. In the days following hatching, the weights of the proventriculus, gizzard, and small intestine increase more rapidly relative to body weight than other organs and tissues. This growth reaches its peak between 4 and 8 days of age, followed by a relative decline. The mass of the small intestine increases nearly sixfold within the first 7 days. It has been observed that the relative intestinal weight increases fourfold from hatching to 4 days of age, and that the maximum proportional weight of the digestive organs is achieved between 3 and 8 days. The intestinal weight then decreases from 7 to 21 days of age. Iji et al. reported that the relative weight of the GIT and digestive organs exceeds overall body weight gain during the early life period, with peak intestinal weight achieved between days 7 and 14. The length of the small intestine and its individual segments also increases with age.
Nitsan et al. [27] found that the relative weights of the duodenum, jejunum, and ileum reached their maximum at 6 days of age and declined thereafter. Similar results were reported by Nir et al. [4], who observed that the maximum relative weight of the small intestine occurred at 5 days of age, and by Nitsan et al. [3], who showed that the maximum relative growth rate was four times that of body weight gain at 8 days of age. Iji et al. [28] and Murakami et al. [29] also demonstrated that the relative weight of the small intestine peaked at 7 days of age and decreased afterward. Ravindran et al. [40] found that the relative weights of the intestinal segments (duodenum, jejunum, and ileum) were at their highest during the first and second weeks of life and declined rapidly thereafter. These observations support the premise that the accelerated development of supply organs immediately after hatching is a prerequisite for sustained post-hatch muscle growth in fast-growing broilers. However, intestinal mass, measured in grams of tissue per centimeter of tissue, continues to increase from hatching up to 35 days of age. These findings indicate that although the relative size of the intestine decreases with age, this decrease is compensated by an increase in intestinal mass to support the nutrient supply function to the required tissues.
The gizzard is known as the “driver” of normal intestinal motility. In addition to contributing to grinding action, increased gizzard activity allows for greater gastric and/or intestinal reflux [41], thereby enhancing the mixing of digesta with enzymes and improving nutrient digestion. At hatching, the gizzard is the largest organ associated with the GIT and is even larger than the liver (52 vs. 33 g/kg body weight). However, the relative weight of the gizzard continues to decrease with age [40].
Nitsan et al. [3] showed an increase in the relative weight of the pancreas up to 8 days of age, at which point the pancreas had an allometric growth rate approximately four times that of body growth. After 8 days, this rate declined, and by day 23, the pancreas exhibited an allometric growth rate 1.5 times that of body weight. In this study, the liver reached its maximum allometric growth rate of two on day 11, and by day 15, this rate had decreased to a level similar to overall body growth. In a subsequent study, Nitsan et al. [27] demonstrated that the relative weights of the liver and pancreas peaked at 6 and 9 days of age, respectively.
Pinchasov [42] showed that the relative weights of the GIT, liver, and pancreas in broiler chicks increased within the first 24 hours after hatching, regardless of whether the chicks were fed, although the increase was greater in fed chicks. A similar pattern of organ growth was also observed in broiler chicks by Murakami et al. [29].
Maturation of the Intestinal Mucosa
The functionality of the GIT is closely related to its microscopic structure. The architecture of the GIT is not well developed during the first week of life but matures rapidly with age. The dramatic post-hatch increases observed in the weight and length of the small intestine may seem minor when compared to the growth of the intestinal mucosa. Changes in villus height, crypt depth, and submucosal thickness greatly contribute to nutrient absorption to meet the needs of newly hatched chicks. Increases in the length and diameter of intestinal segments also significantly affect the surface area available for absorption.
Uni et al. [44] found that villus height and surface area increased rapidly, at varying rates across the three intestinal segments, by 25–100% between days 4 and 10 post-hatch, with the most significant increases observed in the jejunum and ileum. Crypt depth, which reflects enterocyte activity, also increased up to day 10. The rate of maturation rose linearly in these segments until day 10. It was observed that villus height and circumference increased by 34% to 100% in all segments of the small intestine between days 4 and 10. Crypt depth and the number of enterocytes per villus also increased with age. Similarly, Uni et al. [45] reported that jejunal villi and crypt development occurred rapidly within 4 to 5 days after hatching, with most epithelial cells proliferating during this period. Noy and Sklan [5] reported that the highest rate of duodenal villus growth occurred around or before 4 days of age, whereas the growth rates of jejunal and ileal villi peaked at day 10.
Uni et al. [38] observed that villus volume in the duodenum, jejunum, and ileum increased with age, with the greatest increase occurring in the duodenum and the smallest in the ileum. The number of enterocytes per villus was similar across all segments and changed little with age. Crypt depth increased two- to threefold as the chicks aged and was greatest in the duodenum.
Iji et al. [28] found that although the intestinal mucosa is structurally present at hatching, it matures rapidly with age through early rapid cell proliferation, hypertrophy, and increased migration rates. The rate of cell proliferation peaks at 7 days of age, while cellular migration reaches its peak at 14 days. The increase in cell proliferation likely supports the growth of crypts and villi. Geyra et al. [46] discovered that all cells along the villi in all intestinal segments are proliferating at hatch. Cell proliferation is sensitive to feed deprivation; however, after refeeding, cell proliferation increases rapidly.
Continued genetic selection for faster growth has been accompanied by changes in the development and architecture of the GIT. Yamauchi and colleagues at Kagawa University, Japan [47, 48], studied the development and maturation of intestinal segments from various chicken strains in a series of experiments. Their work confirmed clear differences between fast-growing broilers and slow-growing layer chicks, with broilers having longer, heavier intestines with greater surface area. Uni et al. [49] reported similar differences between heavy (Arbor Acres) and light (Lohman) strains in intestinal development. Uni et al. [44] characterized the post-hatch intestinal morphology of these two strains in parallel with enzyme secretion, transit time, and digestion. Villus volume and enterocyte density were greater in the heavy strain than in the light strain at hatch, and the rate of change with age was similar in both strains. Enzyme secretion per gram of feed intake into the duodenum was higher in the heavy strain on day 4 post-hatch, but no difference was observed thereafter. Retention time was 50% shorter in the light strain on day 4, but the difference was no longer significant after day 10. Other researchers [27, 50] reported similar differences and trends in enzyme secretion between low- and high-body-weight strains.
In summary, the growth of the intestinal mucosa through cell proliferation and hypertrophy occurs rapidly during the first week of life. The rate of growth varies among intestinal segments, but all reach their maximum developmental rate between days 7 and 14.
Digestion and Nutrient Utilization
The results of complex developmental changes in the anatomical structure, physiology, and function of the GIT in newly hatched broiler chicks are reflected in nutrient digestibility and energy utilization. It should be noted that most published data on nutrient digestibility in very young chicks are measured at the level of the total GIT, which can be influenced by contributions from urinary nutrients and the modifying actions of hindgut bacteria [73]. For this reason, measuring digestibility at the ileal level is preferred; however, this presents challenges due to the low feed intake in chicks, which results in insufficient ileal digesta for laboratory analysis.
Several studies have investigated nutrient digestibility during the first few weeks after hatching. Starch digestion is considered non-limiting in newly hatched chicks [74, 75]. Chicks hatch with some amylase reserves that accumulate in the pancreas during the final days of embryonic development [4, 61], and they adapt well to starch digestion upon hatching. Amylase activity appears to mature more rapidly than other digestive enzymes.
Although the yolk contains less than 1% carbohydrate and newly hatched chicks have not yet ingested any feed, the intestinal mucosa at hatch exhibits high levels of disaccharidase activity [38]. Noy and Sklan [66] found that net duodenal secretion of amylase, trypsin, and lipase was low at 4 days of age but increased approximately 100-, 50-, and 20-fold, respectively, by 21 days. Total-tract starch digestibility increased rapidly during the first few days after hatching, reaching 97% by days 4 and 8 of life in layer- and broiler-type chicks, respectively [76]. High starch digestibility values of 85–95% have also been observed in chicks in other studies [66, 77]. However, the assumption of nearly complete starch digestibility at hatch can be challenged, as such measurements are taken across the entire tract and may be influenced by the modifying actions of hindgut bacteria. On one hand, as mentioned earlier, it is difficult to obtain sufficient ileal digesta during the first few days post-hatch. Equally important, it should be noted that steady-state transit conditions necessary for ileal digestibility measurements using indigestible markers are achieved only after day 4 post-hatch [9], largely due to very low feed intake.
Optigrin
- Effective: Proven to suppress the growth of pathogenic microbes (an alternative to AGPs)
Optigrin contains carvacrol and thymol, which possess antibacterial and antiprotozoal properties.
Optigrin forms a protective layer on the intestinal villi, preventing sporozoites from penetrating the intestinal wall. Optigrin
inhibits the multiplication of Eimeria. - Contains antioxidants that can neutralize free radicals
Optigrin contains rosmarinic acid, which has strong antioxidant properties - Contains immunostimulants
Optigrin contains andrographolide, which can enhance the function of the body’s defense system—such as white blood cells—to attack bacteria and antigens - Safe, non-residual, and non-resistant
According to FDA data, plant-derived products are generally classified as GRAS (Generally Recognized As Safe), meaning they are considered safe and non-toxic when used as directed










