The Perfect Choice for Your Livestock, Agriculture, and Pet Care Needs
We bring decades of experience and a competent professional team to support your business.
With a vision to be the most trusted primary partner, we continuously expand our distribution network and offer high-quality products supported by professional services. Through our commitment to innovation and mutually beneficial cooperation, Medion Ardhika Bhakti helps increase productivity and the sustainability of our clients' businesses across the country.
Leading Solutions for Your Needs
We provide integrated services designed to meet customer needs, enhance productivity, and maintain health in the livestock, agriculture, and pet care sectors.
Animal Husbandry

Complete solutions for poultry, ruminants, and swine farming. Our offerings include biosecurity products, sanitation solutions, and advanced technology to enhance livestock management.
Agriculture

High-quality agricultural products to optimize productivity. Our commitment is to provide innovative and reliable solutions tailored to your farming needs.
Pet Care

A range of products for the care, medication, and food for dogs, cats, gamefowl, and birds. Designed to support optimal health, immunity, and performance for your beloved pets.
Aquaculture

We offer a range of high-quality aquaculture products to support fish and shrimp health, enabling more productive and sustainable farming. With research-based innovation, we help farmers achieve optimal yields through effective and reliable solutions.
Education, Livestock Services, and Laboratory
We provide educational services, livestock services, and laboratory services designed to increase productivity and meet your needs.
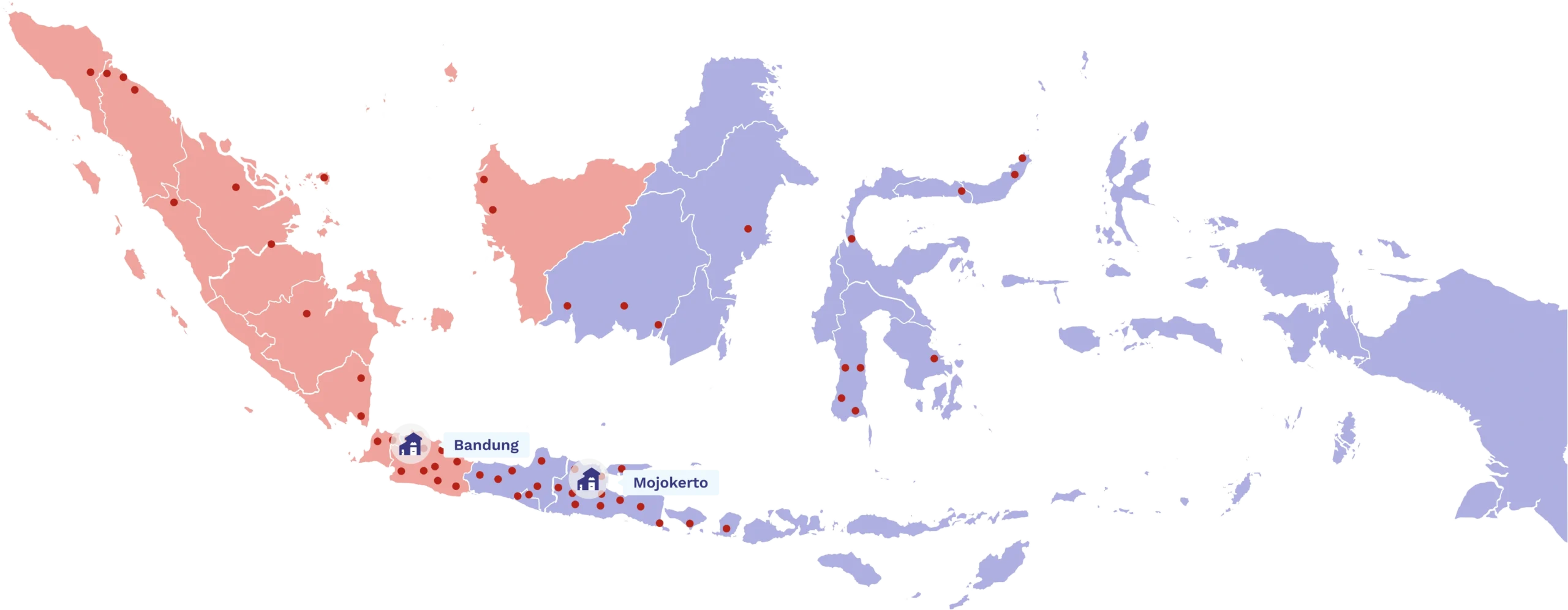
Wide and Reliable Distribution Reach
Medion Ardhika Bhakti ensures quality products are easily accessible throughout Indonesia. As a leading partner in marketing and distribution, we are committed to providing fast, efficient, and trusted services to support your business needs.
Customers
Distribution points across Indonesia
Provinces in Indonesia
Trusted Partners from Various Sectors
Medion Ardhika Bhakti ensures quality products are easily accessible throughout Indonesia. As a leading partner in marketing and distribution, we are committed to providing fast, efficient, and trusted services to support your business needs.






We’re Ready to Help You!
Whatever your needs or questions, our team is always prepared to provide the best solutions.